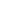Insulation and Air Sealing: Extreme Coffee and Tea Edition

I love analogies. Always have. Always will. So I was thinking about insulation and air sealing the other day as I drank coffee from my fancy-schmancy Contigo coffee mug (ok, they only cost $20, so don’t judge).
I love this mug. It has a perfect air seal and you have to push a button to get it to open up. It has double walled construction with a vacuum between the walls for insulation.
Basically, from an air sealing and insulation perspective, the thing is darn near perfect. Even Joe Lstiburek, patron saint of Building Science, would probably approve. The manufacturer says it will keep your hot beverage hot for up to 4 hours. Like laptop battery life, that’s a bit of a stretch and depends on what you call ‘hot’. Still I’ve found it’s easily good for 2 hours.
I have a few other mugs too, plus my wife and I like to drink tea with dinner, so we have a few different tea pots as well, including a new one my wife got for Christmas that has a knitted tea cozy. (She must have been British in a former life.)
Aha! An experimental analogy to see how insulation and air sealing affect how hot coffee and tea stay using different vessels! Sheer genius! (It was one of those high on yourself kind of moments.) Has anyone ever come up with an experimental analogy before? Brilliant!
Seriously, I thought this would be a totally different angle to come at insulation and air sealing. My winter coat analogy where the coat is insulation and the zipper is air sealing is not bad, but I always like to find a different way to get my point across. Both are important, but air sealing is usually left out of the equation. It turned out it made an even bigger difference than I was expecting. But now I’m getting ahead of myself.
The Players & Their Attributes
From nearest to farthest with their chart labels in () :
1. Contigo 16 oz. Autoseal West Loop Stainless Steel Travel Mug (Contigo) – Well air sealed with a double walled vacuum core.
2. Ceramic Double Walled No Vacuum Mug from AMG Marketing Resources (Ceramic) – 2 small air holes on top. Decent insulation, presumably.
3. Design for Living Double Wall Travel Mug (Plastic)- 2 small air holes on top, not vacuum sealed.
4. Target Early Bird Coffee Mug (Mug) – plain old large mouthed cup
5. Small Target Teapot with Tea Cozy (Tea Cozy) – the insulation on this is basically a hand knitted winter hat, no air seal.
6. IKEA Varme Teapot (Ikea Pot)- no insulation or air seal. It has about twice the capacity of the tea cozy pot.
The first 4, the mugs, are a fairly direct comparison between an air sealed and insulated vessel, 2 insulated vessels, and an uninsulated and unairsealed vessel.
The last 2, the teapots, are a comparison between insulated and not insulated. I feel bad the teapots didn’t get much of a glamour shot, so here you go:

The Procedure
If you’re not a detail person, skip to the results below…
Water was boiled and measured about 210-212 degrees. Room temperature was about 65 degrees.
I then put approximately 1 cup of water in each container. This wasn’t as precise as I would like because it was tough to get an even cup of water out of a boiling pot.
Finally, I started measuring temperatures. It took a few minutes per measurement to take all the readings, but I did them in the same order every time, so it shouldn’t make a huge difference in the results. I would say don’t try this at home, folks, but that’s exactly what I did. So if the results are off by a degree here and there, realize this is half-butt science.
I also rounded temperature readings to the nearest degree and tried to use the ‘max’ setting on the infrared gun to find the hottest point for each reading. This made about 2 degrees difference most of the time until the last few readings.
OK Enough Preamble! Time For Results!
| Time Elapsed | Contigo | Ceramic | Plastic | Mug | Tea Cozy | Ikea Pot |
| 3:00 | 179 | 174 | 172 | 158 | 164 | 147 |
| 7:00 | 167 | 159 | 153 | 131 | 143 | 123 |
| 15:00 | 165 | 154 | 151 | 126 | 141 | 120 |
| 20:00 | 163 | 150 | 144 | 115 | 135 | 112 |
| 30:00 | 157 | 139 | 135 | 100 | 125 | 95 |
| 45:00 | 148 | 124 | 122 | 89 | 115 | 92 |
| 60:00 | 141 | 114 | 112 | 83 | 105 | 86 |
| 1:15:00 | 134 | 103 | 103 | 77 | 99 | 82 |
| 1:40:00 | 124 | 92 | 92 | 71 | 89 | 76 |
| 2:30:00 | 115 | 82 | 84 | 68 | 82 | 74 |
| 3:30:00 | 103 | 76 | 77 | 66 | 76 | 71 |
Lessons Learned
First off, if you’re still reading, you are a fellow geek. Congratulations, isn’t it great that it’s cool to be a geek right now? (By the way, see the new 21 Jump Street.)
Past 1:40, everything but the Contigo had essentially cooled, so I am focusing on the 1:40 readings.
1. Air sealing AND insulation kick some serious butt. So much for geekery. At 1:40, the Contigo mug outperformed the regular mug by 75%!*
2. Air sealing kicks insulated butt too, outperforming the 2 insulated mugs by 35% at 1:40.
3. Insulation still helps a lot too, the insulated mugs outperformed the plain old mug by 30% at 1:40.
4. Materials and double walls don’t have a big impact on effectiveness. The insulated mugs and the insulated tea pot performed about the same. At 1:40 the temperatures were effectively the same. I was surprised that the plastic double walled mug didn’t perform better than ceramic. So their R-value is essentially the same.
5. Using a big teapot was not a great idea, it’s starting temperature was so low because of its large mass as to be useless for comparison. Perhaps the lesson is that big houses suck lots of heat?
So there you go, insulation is good, but insulation AND air sealing are far better. As illustrated by a blogger left home alone while his wife went to roller derby practice. Tell me what you think of my nutty entry below!
* Yes, I know this is Fahrenheit and is not based on absolute zero so the value of percentages is a bit dubious, it doesn’t really matter with room temperature at 65 for the point of comparison while using rather hare-brained science.
COMMENTS:
I liked this post a lot better than I thought I might, good job…now I think I need a cup of tea..
Your kitchen looks neat, and you pictures draw the viewer in.
I loved that you mentioned mass because I instantly thought of thermal inertia, and how you might further squeeze bias from the test. Wanna do the test again?
This time, heat all the containers. Fill them with boiling water, leave for 1-2 minutes, THEN put the cup of boiling water in and start timing.
I wonder if the results will uncover anything interesting.
I like TedKidd’s suggestion about warming up the containers first to account for the thermal mass of the different materials. Isn’t that a general rule of teapot use for that very reason? ;>)
David, I hadn’t even thought of the thermal bridging effect, you are absolutely correct. Thermal bridging is a very important consideration in insulating any envelope. The Contigo is only OK in this regard, there is no insulation at the top of the metal mug where the cap screws on, so it has a thermal bridge as well. So much for it’s perfection! =)
I may rerun the experiment sometime. It was pretty quickly set up on a whim, and I did warn it’s half-butt science!
Get the HVAC Guide

It's free! Make buying a new furnace, air conditioner, or heat pump less stressful.