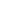Can ventless fireplaces make too much carbon dioxide?
It’s Energy Smart Blog’s first guest entry! My old friend Steve Driver explores how much carbon DI-oxide is produced by ventless fireplaces, and if that could be a hazard above safe levels prescribed by OSHA and the like.
Introduction

Sometimes, it’s a small world. Steve Driver, my weightlifting partner in high school and classmate in advanced math and science classes turned out to be a professional engineer and LEED AP with Siemens in New York City. What are the odds?
We reconnected recently and figured out we do very similar things. He was bothered by an article on Energy Vanguard about ventless fireplaces, written by another good friend, Allison Bailes. As one of 2 people who got a higher grade than me in AP Chemistry, (possibly the most brutal class in high school I took, only 3 A’s out of 25 or so smart kids, I had one of them) Steve likes to run experiments. He doesn’t deny that ventless fireplaces aren’t the best idea in the world, but he doesn’t want to see them thrown out without a little more scientific evidence. So he offered to write a guest post for me about his calculations with carbon dioxide emissions from ventless fireplaces. This does not heavily address moisture, soot, etc, just CO2. Please note, he addresses carbon monoxide (CO), the stuff with the severe side effect of death, but his focus is on the more standard CO2 found everywhere. Take it away, Steve!
Show Your Math
Recently a blog post about the potential liability of ventless fireplaces caught my eye. The reason it did is that it provided some science on the issue but no serious engineering calculations. Whenever I see one of these articles I always want to ask for a calculation, simulation or other math that produces a real conclusion. Then I thought… I could figure out exactly how bad one of these could be, or not be.
I will assume if you are reading this you have read Allison’s article. I looked online to check out typical size units. A simple Google search found many available models ranging as high as 36,000 BTU/hr. The most common fuel to use in these is natural gas, which is about 1000 BTU per cubic foot (others may use propane or ethanol). The average size is about 25,000 BTU/hr. These sizes are capacities and they can be operated at lower heat rates. For this analysis I used a typical 25k unit. For natural gas, the vast majority of fuel is CH4 (methane) which burns in the following equation: CH4 + 2O2 -> CO2 + 2H2O. So for each cubic foot of gas burned produces about 1 cubic foot of CO2.
Any combustion process can also produce CO (carbon monoxide), which I will not be looking at. These units offer a high excess air combustion environment, and it is difficult to predict the amount of CO, and nearly every house has a CO alarm. If yours doesn’t, get one before finishing reading this article. Seriously, go to the hardware store right now. The website will be here when you get home.
There are several aspects that determine the CO2 concentration. 1) Fuel burn rate, volume of the living space, natural and forced ventilation rate (how leaky is the house and any exhaust fans), and the amount of runtime. This reaches an equilibrium point typically after about 10-12 hours, when the production of CO2 in the fireplace is equal to the natural ventilation times difference of the inside and outside concentrations.
Standards for CO2 Concentration
There are standards on outside ventilation for commercial buildings set by ASHRAE. CO2 is used as a feedback to indirectly measure this ventilation rate, and the limits provide a method to measure ventilation without having to measure a compressible gas flow at potentially many different locations, with instruments that would require costly calibrations on a annual basis.
These limits are set due to fact that as CO2 concentrations rise, so too do other “off” gases (from drywall, ceiling tiles, carpeting, and people), and using CO2 as a bell-weather for other gases works well to maintain a good working environment so employees can be comfortable and keep sharp focus on their work and be productive. These limits (800 to 1200ppm) are far lower than “safe” CO2 levels set by OSHA and NIOSH.
OSHA and NIOSH limits are about 5000 ppm (0.5%) for long-term exposure (40 hours per week), and a 30,000 ppm short-term limit (15-min). 40,000 ppm is considered instantly dangerous. But their enforcement is focused less on CO2 and more on a minimum level of Oxygen (19.5%), generally in permit-required confined spaces. If O2 is displaced only by CO2, a level of 14,000 ppm is required to reach 19.5% O2. These measurements are for “dry” air so any amount of water vapor is removed from the calculation. Water vapor could be as high as 1.6% by weight, and 2.6% by volume in 70F room temperature air, so the limit of CO2 would be 13636 ppm to maintain 19.5% O2 in completely saturated air.
Apollo 13 & Computation Methodology
One of the more famous CO2 “events” was the Apollo 13 mission, where levels approached 20,000 ppm (2%) before the jury-rigged scrubber was installed (1). The movie added some suspense that they would suffocate at any moment. They were able to focus and work methodically through this concentration, but they were likely feeling some effects by the time they finished.
To calculate (or simulate) the CO2 concentration over time, I used the following assumptions.
1) 25,000 BTU/hr unit running at full load, 25 CF of CO2 generated per hour
2) 2000 square foot house with 8 foot ceilings (16,000 cubic feet total volume)
3) 0.375 ACH (air changes per hour) natural ventilation, which is a typical average of a home (range is 0.22 to 0.75). For the above 16,000CF house, this is 100CFM or 6000CFH. This does not include any additional forced ventilation (such as a kitchen or bathroom exhaust fan).
4) 4 occupants in the home. Each occupant provides an additional 1 CF of CO2 per hour, giving a total CO2 rate of 29CF/hr.
5) Outside CO2 concentration of 380ppm.
There are two ways to make the calculations, explicitly or recursively. The explicit method requires determining a function for the differential change in CO2 concentration (dC/dt) and using Calculus to integrate over the time period. The recursive method requires doing a few thousand calculations. In the old days, calculus would have been the preferred choice. But today, a spreadsheet program like MS Excel can handle this task easily and we can adjust the inputs to look at different situations.
Regardless of size, a house with 29CFH of CO2 production and 6000CFH of outside air movement has an equilibrium concentration of 5213ppm. This is (29/6000)*10^6+380. The amount of CO2 production is the burner output (in KBTU/hr) plus the number of occupants.
This is just over the long-term limit set for working conditions enforced by OSHA/NIOSH, it is also the typical level found in manned spacecrafts during orbit (1). The size of the house determines the time required to get to equilibrium. For the 2000 sq ft house it takes about 8 hours. Without the fireplace and with everyone in the house, the equilibrium concentration is 1047ppm (4/6000)*10^6+380. Over a 24 hour period (8 hours on, 16 hours off, occupied by 4 people 14 hours per day), the average exposure was 3225 ppm (during the 14 hours per day of occupancy).
Humidity is another topic of discussion, the equilibrium humidity ratio of the house could get to 0.006 above the outside humidity ratio. For typical cold weather, this would correspond to an inside 55-60% relative humidity (at 70F Db). This is not cause for alarm but depending on your particular house’s condition, condensation may be possible on cold surfaces. Assuming you have efficient windows and decent insulation, you shouldn’t have an issue. For draftier houses, this equilibrium humidity ratio point would be lower.
The following graph simulates the following scenario. At 5:00PM everyone (4) returns home (2000sqft, 0.375 ACH natural ventilation) and the ventless fireplace is turned on. The fireplace automatically shuts off at 1:00AM. Everyone leaves at 7:00AM for work/school/etc, and the house is vacant until 5:00PM when the cycle begins again.
Conclusion:
It is nearly impossible for a ventless fireplace to cause an dangerous (>20,000ppm) level of CO2 concentration in a house, even if left on all the time.
However, since no specific level of CO2 concentration affects everyone the same, one person may feel light headed while others are fine. If a ventless fireplace is used for only a few hours at a time and you turn on a kitchen or bathroom exhaust fan with it, the CO2 concentration can be reduced to much lower levels and no one should feel any effect. And just so no one thinks I forgot about it, the unit should have a remote CO monitor that can automatically shut the unit off before CO levels become dangerous.
The house’s equilibrium CO2 concentration point is determined by the following equation:
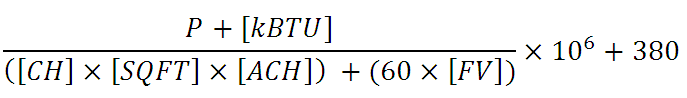
Where:
P = number of people in the house
kBTU = heat rating of the unit in thousand BTU’s per hour (typically 10 to 36), use 0 if not running.
CH = Ceiling Height (typically 8 to 9 ft)
SQFT = area of the house in square feet
ACH = natural ventilation air changes per hour. Tricky to determine, but according to a very good source in the home energy efficiency business, “tight” houses are as low as 0.22, while drafty houses are as high as 0.75. 0.375 is typical.
FV = any additional forced ventilation in cubic feet per minute (CFM), such as bathroom or kitchen exhaust. Fans moving air around the house do not count, only those directly blowing it out or in.
The numerator of the first term is the total cubic feet of CO2 produced per hour and the denominator is the total CF exhausted per hour. 380 ppm is the ambient outside CO2 concentration.
Citations:
1. Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants: Volume 5 (2008)
Board on Environmental Studies and Toxicology (BEST)
Get the HVAC Guide

It's free! Make buying a new furnace, air conditioner, or heat pump less stressful.